 |
A. Photoinitiated Free Radical Polymerization
When polymerizations are initiated by light and both the initiating species and the growing chain ends are radicals, we speak of radical photopolymerization. In far the most cases of photoinduced polymerization, initiators are used to generate radicals. One has to distinguish between two different types of photoinitiators.See animation of the photopolymerization of the different type monomers. Photopolymerization of monofunctional monomers, Photopolymerization of bifunctional monomers
Type I Photoinitiators: Unimolecular Photoinitiators. These substances undergo an homolytic bond cleavage upon absorption of light. Benzoin and its derivatives are the most widely used photoinitiators for radical polymerization of vinyl monomers.

Type II Photoinitiators Bimolecular Photoinitiators. These photoiniating systems consist of a photoinitiator such as benzophenone or thioxanthone and a coinitiator such as alcohol or amine. Radicals are generated in a bimolecular process by the reduction of photoexcited aromatic carbonyl compound by hydrogen abstraction or electron transfer reactions
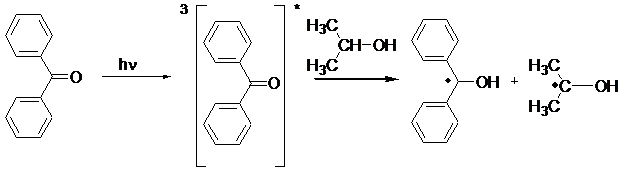
B. Photoinitiated Cationic Polymerization
Direct Acting Systems
Upon photolysis, these thermally and hygroscopically stable initiators undergo irreversible photofragmentation to produce cation radicals and Brønsted acids. Reactive species thus produced photochemically with onium salts initiate the cationic polymerization of suitable monomers as illustrated below.
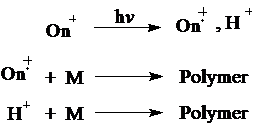
Indirect Acting Systems
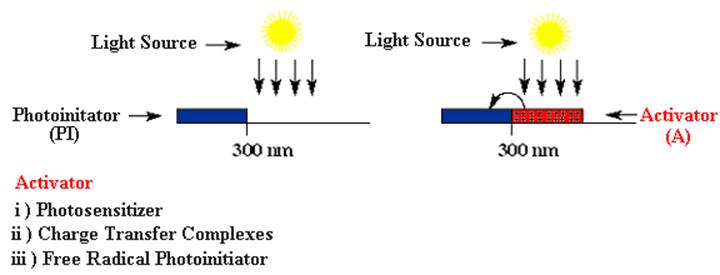
For practical applications, onium salts should absorb light appreciably at wavelengths longer than 350 nm where the commercially available medium and high-pressure mercury lamps emit much of their radiation. The indirect actions of these systems, which extend their spectral sensitivity to longer wavelengths, are shown below..
Several indirect ways to expand wavelength range have been described. All of these pathways involve (i) electron transfer reactions either with photoexcited sensitizer or free radicals (iii) and with the electron donor compounds in the excited charge transfer complexes (ii)
(i) Photosensitizer
Many aromatic hydrocarbons such as anthracene, phenothiazine, and perylene are able to sensitize the decomposition of onium salts via electron transfer. The irradiation of the sensitizer is followed by the formation of a complex between excited sensitizer molecules and ground state onium salt. In this complex, one electron is transferred from the sensitizer to the onium salt giving rise to the generation of sensitizer radical cation as a result of homolytic cleavage of the corresponding onium salt. The radical cations themselves initiate the polymerization of appropriate monomers or, alternatively, interact with hydrogen donor constituents of the polymerization mixture (such as solvent or monomer) resulting in the release of Brønsted acid.
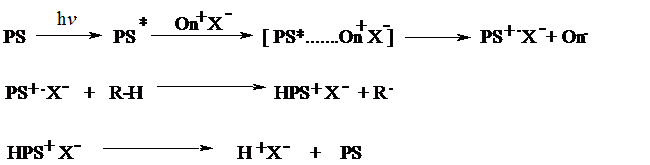
(ii) Charge Transfer Complex
Pyridinium salts are capable of forming charge transfer (CT) complexes with electron rich donors such as methyl- and methoxy-substituted benzene. It was found that the CT complexes formed between pyridinium salts and aromatic electron donors act as photoinitiators for the cationic polymerization of cyclohexene oxide and 4-vinyl cyclohexene oxide. The mechanism illustrated in equations 19 and 20 for the initiation of the cationic polymerization has been suggested.
(iii) Free Radical Promoted Cationic Polymerization
Among the indirectly acting initiating systems, free radical promoted cationic polymerization is the most flexible route, since free radical photoinitiators with a wide range of absorption characteristics are available. Many photochemically formed radicals can be oxidized by onium salts. The cations thus generated are used as initiating species for cationic polymerization according to the following reactions.
