| Controlled Polymerization Methods |
 |
(i) Nitroxide Mediated Polymerization
In NMP systems, stable free radicals, such as nitroxides, are used as reversible terminating agents to control the polymerization process. Dormant chains are generated by reversible deactivation of the growing chains through covalent bond formation. At high temperatures, the bond undergoes a homolytic cleavage to produce the active growing chain and the nitroxide radical. Activation is followed by a rapid deactivation, whereby a few monomer units are incorporated to the propagating chain.
See animation of the NMP
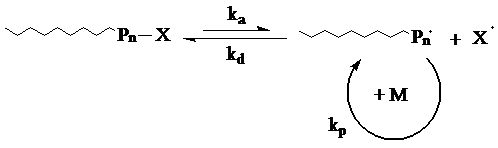
(ii) Atom Transfer Radical Polymerization
Atom Transfer Radical Polymerization, pioneered by Matyjaszewski and Sawamoto, is based on a continuous and reversible halogen transfer (pseudo halogen) between a dormant propagating species, Pn, and a transition-metal (eg. Cu), complexed by a ligand (eg. bipy), in its lower oxidation state (Scheme 6). Halogen transfer is accompanied by a one-electron oxidation of the transition metal, whereby propagation takes place by the addition of monomers to the activated chains. Homogeneity of the polymeric chains is controlled by the fast and simultaneous initiation as well as rapid deactivation of the growing chains. Here, the rate of deactivation should be faster than the propagation rate for an effective control. Low concentration of the active centers, maintained by deactivation, suppresses termination and chain transfer reactions at later stages
See animation of the ATRP
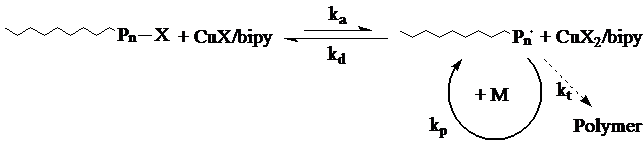
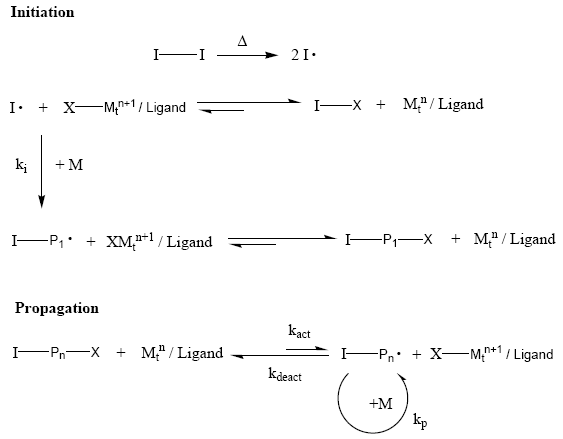
Reverse ATRP differs from ATRP in its initiation process, where a conventional radical initiator, such as AIBN (2,2’-Azobisisobutyronitrile), is used. As shown below, in the initiation step, once generated, the initiating radicals or the propagating radicals, I. Or I-P., can abstract the halogen atom X from the oxidized transition-metal species, XMtn+1, to form the reduced transition-metal species, Mtn, and the dormant species, I-X or I-P1-X. In the subsequent steps, the transition-metal species, Mtn, promotes exactly the same ATRP process as normal ATRP where R-X/Mtn/Lx are used as the initiation system. Instead of first activation of a dormant species, R-X, with Mtn, as in the case of normal ATRP, reverse ATRP originates from the deactivation reaction between radicals, I. or I-P., and XMtn+1.
See animation of the RATRP
(iii) Reversible Addition-Fragmentation Chain Transfer Polymerization (RAFT)
Reversible Addition-Fragmentation Chain Transfer Polymerization (RAFT), developed by Rizzardo and co-workers in the late 1990s, utilizes a chain-transfer- active thiocarbonylthio moiety for the exchange between active and dormant chains. The mechanism is illustrated in below.
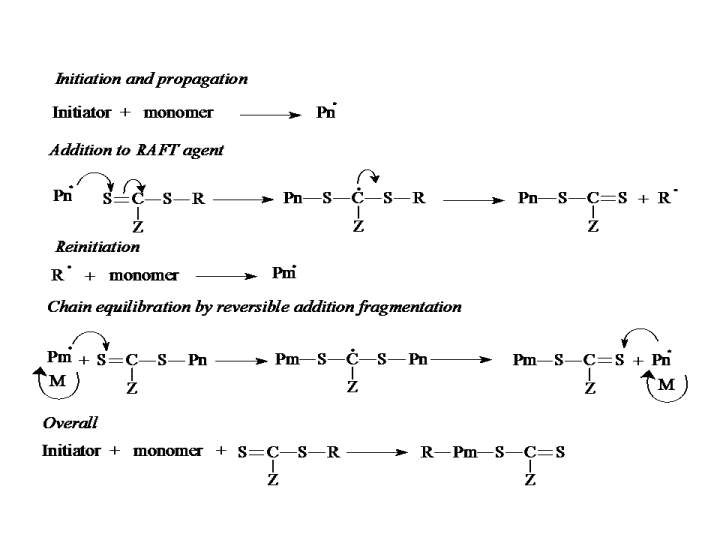
The active species, such as the radicals stemming from the decomposition of the initiator and propagating radicals (Pn.), are transferred to the RAFT-agents, e.g. the thiocarbonylthio moiety. Meanwhile, an intermediate radical is formed, and undergoes a fast fragmentation reaction, giving a polymeric RAFT agent and a new radical. The radical reinitiates the polymerization. The equilibrium is established by successive chain transfer-fragmentation stages. Z and R groups in the thiocarbonylthio compound represent the activating group and homolytically leaving group, respectively. These, in turn, determine the rates of addition and fragmentation. As a matter of fact, the choice of RAFT agent for a specified monomer is rather significant and affects the degree of control.
See animation of the RAFT