| Conjugated Polymers |
 |
T
here is a long interest to prepare soluble conjugated polymers so that polymerization, purification, characterization and processability to be carried out in solution, polymers to be free defects, with a known structure and to form thin films. Owing to their intrinsic chemical structure, conjugated polymers are insoluble in most organic solvents and improving their solubility and processability has been became an important objective that focused many research efforts. From the chemistry viewpoint this disadavantage could be surpassed by design of adequate monomers and introduction of solubilizing side chains onto the conjugated backbone is a very used and efficient method for solubility improving. However, the side substituents used until now are short alkyl chains or other functional substituents for introduction of other supplementary properties; liquid crystallinity, optical activity, ionic groups, etc.The attaching of usual polymer short chains with a well defined length; i.e., polystyrene, polytetrahydrofuran, polylactone and poly-N-acetyl ethylenimine chains, onto polyphenylenenes and polythiophenes and properties of branched copolymers have reported.
Poly(p-phenylene) (PPP) is a typical conjugated,
electroluminescent polymer for light emitting devices in
combination with excellent mechanical properties and thermal and
thermo oxidative stability. The key structural factor in
describing the supramolecular ordering of PPP is their
anisotropic shape, which follows from a rodlike architecture
that differentiates them from flexible polymers. Unfortunately,
PPPs are insoluble in many organic solvents, which limit their
processability. Therefore, attachment of conformationally mobile
alkyl side chains to the backbone has been important because it
has allowed the controlled synthesis of soluble and processable
PPPs with high molecular weight. In view of the expected large
persistence length of the main chain and of the flexibility of
the side chains, such molecules have been termed “hairy-rod”
polymers. On combining a stiff, insoluble, rod-like polymer such
as PPP with a soft coil, for example polystyrene, it is possible
to form a new polymer with novel and interesting properties.
The design process, the essence of which is the chemical control
of size and shape of PPPs, ultimately leads to conjugated
polymers of varying, controlled dimensionality.
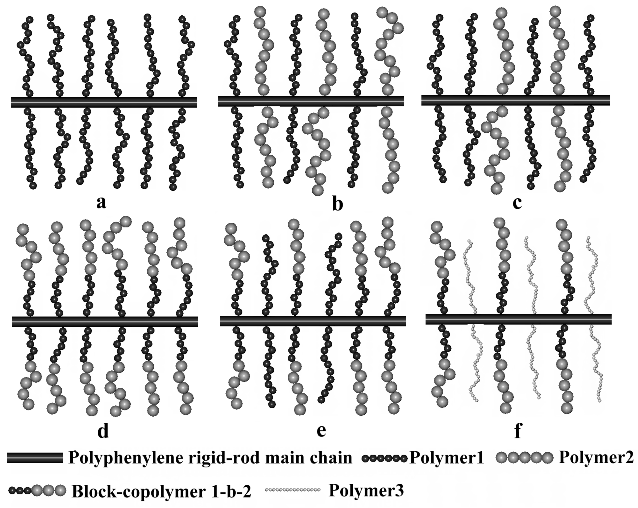
Our studies focused on the synthesis of PPP type graft
copolymers that can present nanostructures between a conductive
and an insulating polymer, by using the macromonomer technique
via controlled polymerizations [ATRP or ROP (Ring Opening
Polymerization)] as versatile “tools”, combined with
metal-catalyzed Suzuki or Yamamoto polycondensation , specific
to the obtainment of soluble, high molecular weight, conjugated
polymers.