| Benzoxazine Based Thermosets |
 |
Among various high performance materials, polybenzoxazine, as a recently developed thermosetting phenolic resin, has received much interest for its unique characteristics such as heat resistance, good flame retardance,
low moisture absorption, good mechanical properties and excellent dimensional stability. Polybenzoxazines are obtained by ring opening polymerization of the corresponding monomers at elevated temperatures without catalysts and releasing by-products according to the following reaction.
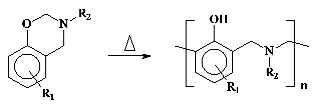
They can also be polymerized by cationic initiators and onium salt photoinitiators. However, in these cases, the structures of the resulting polymers are complex and the properties are different than those prepared by thermal means in the absence of a catalyst. Therefore, the most of the studies on benzoxazines focused on their thermal polymerization.
Benzoxazine monomers as the polybenzoxazine precursors can be easily prepared from inexpensive raw materials like phenols, formaldehyde, and primary amines.
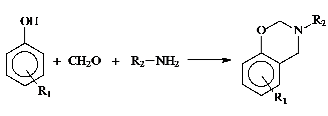
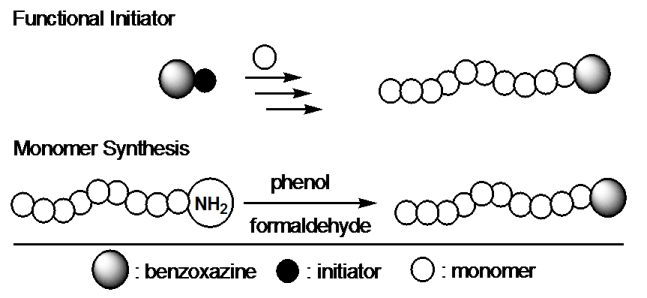
This flexibility provides possibility of synthesizing a wide range of benzoxazine monomers with additional functionalities such as acetylene, nitrile, propargyl and maleimide groups. The incorporation of benzoxazine groups into polymers is an alternative way to further improve the properties. The benzoxazine groups act as thermal crooslinker and while the polymer may be accountable for the flexibility of these materials. Thus, thermally crosslinked non-brittle polybenzoxazine films can be prepared. In this paper, we describe our synthetic approaches proposed to prepare benzoxazine functional polymers. The incorporation of benzoxazine groups into polymers can proceed through two main synthetic routes;
-
Performing polymerization of a particular monomer using a benzoxazine derivative possessing suitable initiating sites (functional initiator).
-
Synthesizing benzoxazine ring structure by using a polymer with amino or phenol groups (monomer synthesis).
The shortcoming associated with the first route is the interaction of some propagating sites, i.e., cations with benzoxazine ring, particularly with nitrogen and oxygen heteroatoms. However, in some polymerization processes propagating species were unreactive towards benzoxazine and the ring structure was preserved during the polymerization. The second route is less sensitive to polymerization conditions but requires functional (mainly amino) polymers in advance. Classical monomer synthesis may successfully be followed with the available amino telechelics.