|
|
|
|
PET 509E Hydrocarbon
Thermodynamics - Fall 2010
Instructor:
Dr. Şenol Yamanlar
Petroleum and Natural Gas Engineering
Department
Component assignments:
505101505 Mehmet A. Torcuk CH4 (Methane)
505101506 Tolga Kilcigil C2H6 (Ethane)
505101507 Yasin Demiralp C3H8 (Propane)
Term Project #2
AD z factor correlation control points
Tr=1.1 Pr=1.2 z=0.5881
Tr=2.0 Pr=6.0 z=0.9857
Tr=2.0 Pr=2.8 z=0.9382
Composite Simpson's Rule for Numerical
Integration
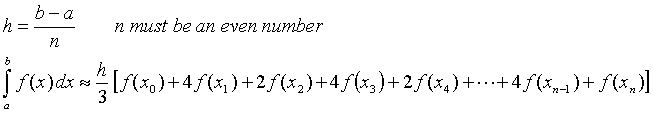
![]()
Code Fragment :
IMPLICIT DOUBLE PRECISION(A-H,O-Z)
N=500
! NUMBER OF INTERVALS
A=0.D0
! LOWER INTEGRAL BOUNDARY
B=10.D0
! UPPER INTEGRAL BOUNDARY
SUM = 0.D0
.............................
DO K = 1, N - 1, 2
! EVALUATE ODD VALUES
PR = A + DBLE(K) * (B - A) / DBLE(N)
SUM = SUM + 4.D0 * F(TR, PR)
ENDDO
DO K = 2, N - 2, 2
! EVALUATE EVEN VALUES
PR = A + DBLE(K) * (B - A) / DBLE(N)
SUM = SUM + 2.D0 * F(TR, PR)
ENDDO
RESULT = (B - A) * ( F(TR, A) + SUM + F(TR, B)) / (3.D0 * DBLE(N) )
! VALUE OF INTEGRAL
........................
DOUBLE PRECISION FUNCTION F(TR,PR)
! FUNCTION TO BE INTEGRATED
IMPLICIT DOUBLE PRECISION (A-H,O-Z)
! F= DZ_DTR (TR, PR) / PR
! IN CASE OF HR
F= 2.D0*PR+1.D0
! A SIMPLE FUNCTION TO TEST THE ALGORIHM
END FUNCTION
| Methane | Ethane | Propane | ||||||||||||||
| T= 1.5Tc | T= 3Tc | T= 1.5Tc | T= 3Tc | T= 1.5Tc | T= 3Tc | |||||||||||
| P | HR | P | HR | P | HR | P | HR | P | HR | P | HR | |||||
| Bar | J/mol | Bar | J/mol | Bar | J/mol | Bar | J/mol | Bar | J/mol | Bar | J/mol | |||||
| 5 | -96.9445 | 5 | -24.0238 | 5 | -148.424 | 5 | -31.6842 | 5 | -207.311 | 5 | -37.7514 | |||||
| 10 | -194.435 | 10 | -49.4122 | 10 | -297.363 | 10 | -63.0767 | 10 | -415.157 | 10 | -75.011 | |||||
| 15 | -292.425 | 15 | -74.43 | 15 | -446.757 | 15 | -93.8956 | 15 | -623.366 | 15 | -111.445 | |||||
| 20 | -390.85 | 20 | -99.1143 | 20 | -596.471 | 20 | -124.22 | 20 | -831.769 | 20 | -147.122 | |||||
| 25 | -489.657 | 25 | -123.431 | 25 | -746.45 | 25 | -154.036 | 25 | -1040.16 | 25 | -182.034 | |||||
| 30 | -588.761 | 30 | -147.32 | 30 | -896.568 | 30 | -183.355 | 30 | -1248.34 | 30 | -216.248 | |||||
| 35 | -688.094 | 35 | -170.882 | 35 | -1046.71 | 35 | -212.19 | 35 | -1456.1 | 35 | -249.713 | |||||
| 40 | -787.553 | 40 | -195.674 | 40 | -1196.76 | 40 | -240.55 | 40 | -1663.17 | 40 | -282.481 | |||||
| 45 | -887.049 | 45 | -216.869 | 45 | -1346.59 | 45 | -268.419 | 45 | -1869.31 | 45 | -314.54 | |||||
| 50 | -986.474 | 50 | -239.359 | 50 | -1496.05 | 50 | -295.792 | 50 | -2074.23 | 50 | -345.896 | |||||
| 55 | -1085.68 | 55 | -261.451 | 55 | -1644.98 | 55 | -322.717 | 55 | -2277.67 | 55 | -376.564 | |||||
| 60 | -1184.55 | 60 | -284.734 | 60 | -1793.26 | 60 | -349.186 | 60 | -2479.35 | 60 | -406.585 | |||||
| 65 | -1282.93 | 65 | -306.151 | 65 | -1940.65 | 65 | -375.209 | 65 | -2678.92 | 65 | -435.914 | |||||
| 70 | -1380.69 | 70 | -327.18 | 70 | -2087.03 | 70 | -400.731 | 70 | -2876.12 | 70 | -464.589 | |||||
| 75 | -1477.64 | 75 | -346.328 | 75 | -2232.19 | 75 | -425.831 | 75 | -3070.61 | 75 | -492.617 | |||||
| 80 | -1573.61 | 80 | -368.268 | 80 | -2375.95 | 80 | -450.484 | 80 | -3262.17 | 80 | -519.966 | |||||
| 85 | -1668.46 | 85 | -388.202 | 85 | -2518.17 | 85 | -474.695 | 85 | -3450.42 | 85 | -546.728 | |||||
| 90 | -1762 | 90 | -406.312 | 90 | -2658.58 | 90 | -498.474 | 90 | -3635.15 | 90 | -572.844 | |||||
| 95 | -1854.05 | 95 | -427.22 | 95 | -2797.05 | 95 | -521.796 | 95 | -3816.06 | 95 | -598.343 | |||||
| 100 | -1944.49 | 100 | -446.227 | 100 | -2933.39 | 100 | -544.708 | 100 | -3992.98 | 100 | -623.242 | |||||
| Methane | Ethane | Propane | ||||||||||||||
| T= 1.5Tc | T= 3Tc | T= 1.5Tc | T= 3Tc | T= 1.5Tc | T= 3Tc | |||||||||||
| P | SR | P | SR | P | SR | P | SR | P | SR | P | SR | |||||
| Bar | J/mol | Bar | J/mol K | Bar | J/mol K | Bar | J/mol K | Bar | J/mol K | Bar | J/mol K | |||||
| 5 | -0.2339 | 5 | -0.045 | 5 | -0.2339 | 5 | -0.0405 | 5 | -0.2766 | 5 | -0.0442 | |||||
| 10 | -0.4704 | 10 | -0.0898 | 10 | -0.4696 | 10 | -0.0806 | 10 | -0.555 | 10 | -0.0878 | |||||
| 15 | -0.7093 | 15 | -0.1342 | 15 | -0.7069 | 15 | -0.1202 | 15 | -0.8353 | 15 | -0.131 | |||||
| 20 | -0.9506 | 20 | -0.1781 | 20 | -0.9458 | 20 | -0.1594 | 20 | -1.1169 | 20 | -0.1736 | |||||
| 25 | -1.1939 | 25 | -0.2216 | 25 | -1.1861 | 25 | -0.1983 | 25 | -1.3998 | 25 | -0.2157 | |||||
| 30 | -1.4392 | 30 | -0.2645 | 30 | -1.4275 | 30 | -0.2367 | 30 | -1.6834 | 30 | -0.2572 | |||||
| 35 | -1.6862 | 35 | -0.307 | 35 | -1.6699 | 35 | -0.2747 | 35 | -1.9675 | 35 | -0.2983 | |||||
| 40 | -1.9346 | 40 | -0.3491 | 40 | -1.913 | 40 | -0.3124 | 40 | -2.2517 | 40 | -0.3389 | |||||
| 45 | -2.1841 | 45 | -0.3908 | 45 | -2.1566 | 45 | -0.3496 | 45 | -2.5356 | 45 | -0.379 | |||||
| 50 | -2.4343 | 50 | -0.432 | 50 | -2.4003 | 50 | -0.3865 | 50 | -2.8188 | 50 | -0.4186 | |||||
| 55 | -2.6851 | 55 | -0.4727 | 55 | -2.644 | 55 | -0.423 | 55 | -3.1007 | 55 | -0.4577 | |||||
| 60 | -2.9358 | 60 | -0.513 | 60 | -2.8871 | 60 | -0.4591 | 60 | -3.3809 | 60 | -0.4964 | |||||
| 65 | -3.1859 | 65 | -0.5529 | 65 | -3.1296 | 65 | -0.4948 | 65 | -3.659 | 65 | -0.5346 | |||||
| 70 | -3.4353 | 70 | -0.5924 | 70 | -3.371 | 70 | -0.5302 | 70 | -3.9343 | 70 | -0.5723 | |||||
| 75 | -3.6832 | 75 | -0.6314 | 75 | -3.611 | 75 | -0.5651 | 75 | -4.2066 | 75 | -0.6096 | |||||
| 80 | -3.929 | 80 | -0.6699 | 80 | -3.8491 | 80 | -0.5998 | 80 | -4.4749 | 80 | -0.6464 | |||||
| 85 | -4.1724 | 85 | -0.7081 | 85 | -4.0851 | 85 | -0.634 | 85 | -4.7393 | 85 | -0.6828 | |||||
| 90 | -4.4128 | 90 | -0.7458 | 90 | -4.3185 | 90 | -0.6679 | 90 | -4.999 | 90 | -0.7187 | |||||
| 95 | -4.6496 | 95 | -0.7831 | 95 | -4.5489 | 95 | -0.7014 | 95 | -5.2537 | 95 | -0.7543 | |||||
| 100 | -4.8823 | 100 | -0.82 | 100 | -4.7761 | 100 | -0.7346 | 100 | -5.503 | 100 | -0.7894 | |||||
Assignment #1 (due 02 December 2010)
Use van der Waals EOS and compute residual properties for H, S, U, and G between P=0 to 100 Bar (ΔP=5 Bar). Do this computations for T= 1.5 Tc and T= 3 Tc for your assigned component.
vdW EOS is cubic in V and Z. To find the gaseous root from the cubic polynomial, use Newton-Raphson iterative sequence, and use an appropriate initial estimate. For Z0, usually an initial estimate of 0.8 to 1.0 will converge to the gaseous root. If you want to solve for molar volume V, then you should calculate a V0 value from PV=RT ideal gas equation of state.
Plot your HR, SR, UR, GR results as a function of P.
Bring your results on a CD to the class, so that you can share your findings with your classmates.
Term Project #1
Assignment #1 (due 28 October 2010)
Use B-truncated virial equation of state (Z= 1 + BP/RT) and write a computer program and plot P vs Z graph for temperatures and pressures given below:
Methane T= 220 K and 300 K (P=0.5 to 200 bar)
Ethane T= 330 K and 400 K (P=0.5 to 200 bar)
Propane T= 420 K and 500 K (P=0.5 to 200 bar)
Predict second virial coefficient from the corresponding states correlation. Use NIST Webbook internet base program to calculate and plot reference curves. Repeat the same tasks using C-truncated virial equation of state. You may solve the cubic equation either for Z or V. Use Newton-Raphson algorithm in your program :
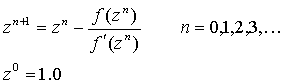
If you choose to use
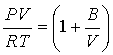
form of the B-truncated virial EOS and solve for V, your program will fail
around 50 bar, since this form of the EOS is only valid at low pressures.
Assignment #2 (due 04 November 2010)
Use B-truncated virial equation of state (Z= 1 + BP/RT) and write a computer program to compute z factor of equimolar binary mixtures of n-Butane.
505101505 Mehmet A. Torcuk CH4 - nC4H10 (Methane+n-Butane) @ 400 K
505101506 Tolga Kilcigil C2H6 - nC4H10 (Ethane+n-Butane) @ 450 K
505101507 Yasin Demiralp
C3H8 - nC4H10 (Propane+n-Butane)
@ 500 K
Reference data for each binary mixture is given below:
| 0.5 C1 + 0.5 n-C4 | 0.5 C2 + 0.5 n-C4 | 0.5 C3 + 0.5 n-C4 | |||||
| T= 400 K | T= 450 K | T= 500 K | |||||
| P | Z | P | Z | P | Z | ||
| Bar | Bar | Bar | |||||
| 0 | 1.000 | 0 | 1.000 | 0 | 1.000 | ||
| 5 | 0.980 | 5 | 0.979 | 5 | 0.980 | ||
| 10 | 0.959 | 10 | 0.958 | 10 | 0.960 | ||
| 15 | 0.938 | 15 | 0.936 | 15 | 0.940 | ||
| 20 | 0.916 | 20 | 0.914 | 20 | 0.920 | ||
| 25 | 0.895 | 25 | 0.891 | 25 | 0.899 | ||
| 30 | 0.872 | 30 | 0.868 | 30 | 0.878 | ||
| 35 | 0.850 | 35 | 0.845 | 35 | 0.857 | ||
| 40 | 0.827 | 40 | 0.822 | 40 | 0.836 | ||
| 45 | 0.805 | 45 | 0.798 | 45 | 0.815 | ||
| 50 | 0.782 | 50 | 0.774 | 50 | 0.794 | ||
| 55 | 0.759 | 55 | 0.750 | 55 | 0.773 | ||
| 60 | 0.737 | 60 | 0.726 | 60 | 0.752 | ||
| 65 | 0.716 | 65 | 0.703 | 65 | 0.732 | ||
| 70 | 0.695 | 70 | 0.681 | 70 | 0.713 | ||
| 75 | 0.676 | 75 | 0.660 | 75 | 0.695 | ||
| 80 | 0.659 | 80 | 0.641 | 80 | 0.678 | ||
| 85 | 0.644 | 85 | 0.624 | 85 | 0.663 | ||
| 90 | 0.631 | 90 | 0.610 | 90 | 0.650 | ||
| 95 | 0.621 | 95 | 0.598 | 95 | 0.640 | ||
| 100 | 0.613 | 100 | 0.589 | 100 | 0.632 | ||
| 105 | 0.607 | 105 | 0.583 | 105 | 0.626 | ||
| 110 | 0.604 | 110 | 0.580 | 110 | 0.622 | ||
| 115 | 0.602 | 115 | 0.578 | 115 | 0.620 | ||
| 120 | 0.602 | 120 | 0.579 | 120 | 0.620 | ||
| 125 | 0.603 | 125 | 0.581 | 125 | 0.622 | ||
| 130 | 0.606 | 130 | 0.584 | 130 | 0.625 | ||
| 135 | 0.609 | 135 | 0.588 | 135 | 0.629 | ||
| 140 | 0.613 | 140 | 0.594 | 140 | 0.633 | ||
| 145 | 0.618 | 145 | 0.600 | 145 | 0.639 | ||
| 150 | 0.624 | 150 | 0.607 | 150 | 0.646 | ||
| 155 | 0.631 | 155 | 0.614 | 155 | 0.653 | ||
| 160 | 0.637 | 160 | 0.622 | 160 | 0.660 | ||
| 165 | 0.645 | 165 | 0.630 | 165 | 0.668 | ||
| 170 | 0.652 | 170 | 0.638 | 170 | 0.676 | ||
| 175 | 0.660 | 175 | 0.647 | 175 | 0.685 | ||
| 180 | 0.668 | 180 | 0.656 | 180 | 0.694 | ||
| 185 | 0.677 | 185 | 0.665 | 185 | 0.703 | ||
| 190 | 0.685 | 190 | 0.675 | 190 | 0.713 | ||
| 195 | 0.694 | 195 | 0.684 | 195 | 0.722 | ||
| 200 | 0.703 | 200 | 0.694 | 200 | 0.732 | ||
Assignment #3 (due 11 November 2010)
Use B-truncated and C-Truncated virial equation of state and compute z factor of n-Butane at your assigned temperatures ( in case of methane there will be condensation at 400 K. To stay in the single phase region use 450 K instead). Plot the results. Use NIST Webbook internet base program to calculate the reference curve and then plot P-Z curve on the same graph.
Compute and plot your assigned component and n-Butane mixtures at your assigned temperature and composition in the reference curves. The reference curves are given below:
| 0.9 C1 + 0.1 n-C4 | 0.3 C1 + 0.7 n-C4 | |||
| 400 K | 400 K | |||
| P | Z | P | Z | |
| Bar | Bar | |||
| 0 | 1.000 | 0 | 1.000 | |
| 10 | 0.991 | 10 | 0.933 | |
| 20 | 0.982 | 20 | 0.858 | |
| 30 | 0.974 | 30 | 0.774 | |
| 40 | 0.966 | 40 | 0.679 | |
| 50 | 0.958 | 50 | 0.572 | |
| 60 | 0.952 | 60 | 0.475 | |
| 70 | 0.945 | 70 | 0.425 | |
| 80 | 0.940 | 80 | 0.413 | |
| 90 | 0.934 | 90 | 0.418 | |
| 100 | 0.930 | 100 | 0.433 | |
| 110 | 0.926 | 110 | 0.453 | |
| 120 | 0.923 | 120 | 0.474 | |
| 130 | 0.921 | 130 | 0.498 | |
| 140 | 0.919 | 140 | 0.522 | |
| 150 | 0.918 | 150 | 0.547 | |
| 160 | 0.918 | 160 | 0.572 | |
| 170 | 0.919 | 170 | 0.597 | |
| 180 | 0.920 | 180 | 0.622 | |
| 190 | 0.922 | 190 | 0.647 | |
| 200 | 0.925 | 200 | 0.673 | |
| 0.9 C2 + 0.1 n-C4 | 0.1 C2 + 0.9 n-C4 | |||
| 450 K | 450 K | |||
| P | Z | P | Z | |
| Bar | Bar | |||
| 0 | 1.000 | 0 | 1.000 | |
| 10 | 0.977 | 10 | 0.930 | |
| 20 | 0.955 | 20 | 0.854 | |
| 30 | 0.932 | 30 | 0.766 | |
| 40 | 0.910 | 40 | 0.664 | |
| 50 | 0.888 | 50 | 0.543 | |
| 60 | 0.868 | 60 | 0.428 | |
| 70 | 0.847 | 70 | 0.384 | |
| 80 | 0.829 | 80 | 0.385 | |
| 90 | 0.811 | 90 | 0.402 | |
| 100 | 0.795 | 100 | 0.424 | |
| 110 | 0.782 | 110 | 0.449 | |
| 120 | 0.770 | 120 | 0.475 | |
| 130 | 0.761 | 130 | 0.502 | |
| 140 | 0.755 | 140 | 0.529 | |
| 150 | 0.751 | 150 | 0.556 | |
| 160 | 0.750 | 160 | 0.583 | |
| 170 | 0.751 | 170 | 0.611 | |
| 180 | 0.754 | 180 | 0.638 | |
| 190 | 0.759 | 190 | 0.665 | |
| 200 | 0.765 | 200 | 0.692 | |
| 0.9 C3 + 0.1 n-C4 | 0.1 C3 + 0.9 n-C4 | |||
| 500 K | 500 K | |||
| P | Z | P | Z | |
| Bar | Bar | |||
| 0 | 1.000 | 0 | 1.000 | |
| 10 | 0.969 | 10 | 0.950 | |
| 20 | 0.938 | 20 | 0.898 | |
| 30 | 0.907 | 30 | 0.844 | |
| 40 | 0.876 | 40 | 0.786 | |
| 50 | 0.846 | 50 | 0.727 | |
| 60 | 0.816 | 60 | 0.667 | |
| 70 | 0.788 | 70 | 0.613 | |
| 80 | 0.762 | 80 | 0.572 | |
| 90 | 0.740 | 90 | 0.550 | |
| 100 | 0.721 | 100 | 0.543 | |
| 110 | 0.707 | 110 | 0.547 | |
| 120 | 0.697 | 120 | 0.558 | |
| 130 | 0.693 | 130 | 0.574 | |
| 140 | 0.693 | 140 | 0.592 | |
| 150 | 0.697 | 150 | 0.612 | |
| 160 | 0.704 | 160 | 0.634 | |
| 170 | 0.713 | 170 | 0.656 | |
| 180 | 0.724 | 180 | 0.679 | |
| 190 | 0.737 | 190 | 0.702 | |
| 200 | 0.751 | 200 | 0.726 | |